|
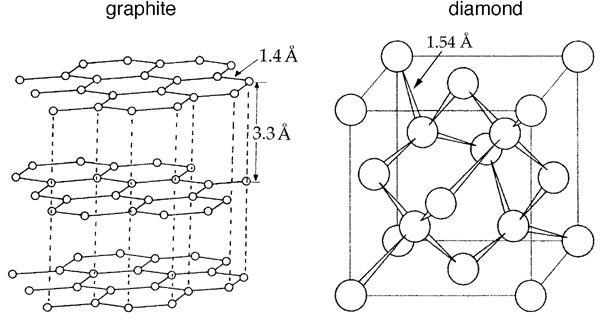
For many hundreds of years, diamond and graphite (Figure 1) were the only known crystalline allotropic forms of carbon. The discovery in the 1980’s of fullerenes, a family of crystalline Cn compounds (with n even), was rewarded with the 1996 Nobel Prize for Chemistry to Robert F. Curl Jr., Sir Harold W. Kroto and Richard E. Smalley (see the web page here).
Figure 1
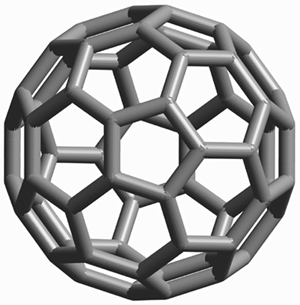
The most prominent of these are fullerenes C60 (Figure 2) and C70. Following the first preparation of fullerenes in macroscopic amounts[1] and the development of high-yield preparative procedures,[2] fullerenes have been subjected to intensive scrutiny. Their unique structure and properties suggest potential applications in materials science (for the production of superconductors, superhard phases and synthetic diamond), synthetic chemistry and medicine (targeted cancer therapy).
Figure 2
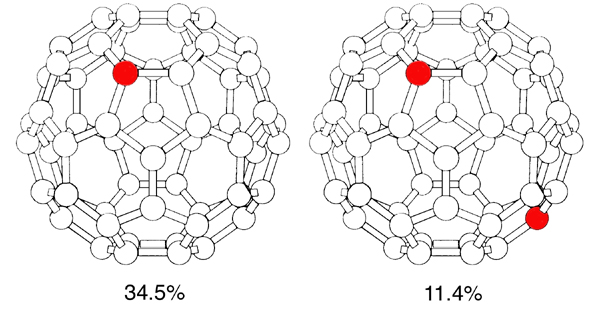
A 13C MAS NMR spectrum of carbon soot produced in the course of fullerene synthesis shows that both C60 and C70 fullerenes are clearly present. 1H MAS NMR detects dilute H-containing species in purified C60.[3] We have identified various fulleroids containing –HC=CHCH2CH3, –HC=CHCH3 and –HC=CH– groups, as well as toluene and dioctylphtalate (DOP) impurities. The H-containing species are so dilute that they cannot communicate by intermolecular spin diffusion. The intramolecular spin-diffusion paths suggest that the alkyl chains of DOP are folded back towards the aromatic ring. Cross-polarization in toluene-solvated C70 at natural 13C isotopic abundance can proceed in both directions (13C to 1H and 1H to 13C) and is not site-specific, which means that each carbon site experiences the same average dipolar field from remote protons during the cross-polarization contact.[4] 2H NMR detects water in two distinct environments in C60 saturated with D2O under external pressure: intercalated large clusters and monomers/small multimeric species.[5] At room temperature the fullerene molecules in solid C60 are close packed and bound by van der Waals forces.[6] Fast cage motion averages the chemical shift anisotropy (CSA) at room temperature and the 13C spectrum of the static sample is only 220 Hz wide. Fast uniaxial averaging would decrease the CSA in comparison with the “no motion” case, to produce an asymmetric line 2 kHz wide, but could not narrow the line to the observed value. 13C NMR T1 relaxation time in pure C60 decreases from 31 to 0.8 s at the phase transition near 262 K as the temperature is decreased by less than 8 K. Static NMR lineshapes are unchanged in the 280–235 K range, but the isotropic 13C chemical shift changes linearly with temperature. We account for the results by noting that the C60 molecules which are observable to NMR (those containing 13C) are by their nature asymmetric tops (Figure 3). 51.3% of all C60 molecules contain no 13C nuclei; 34.5% of the cages contain just one 13C atom and 11.4% two. “NMR-visible” molecules undergo gyroscopic motion involving rotation, precession and nutation. Precession of a sphere cannot account for the narrow 13C resonance from the static sample, and nutation must be present to account for the substantial averaging of the CSA.
Figure 3
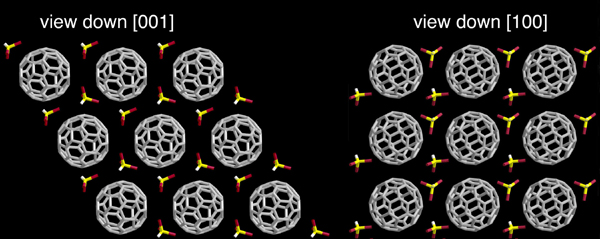
Production of fullerenes involves their dissolution, while their functionalization is performed in a variety of aromatic solvents. Solvation is thus a crucial step, and the understanding of intermolecular interactions of fullerenes with solvent molecules is essential for the rationalization of their properties. In particular, knowledge of the solubility of fullerenes in organic solvents, and of the temperature dependence of this process, is important for isolating fullerenes and their derivatives. Solvates of C60 and C70 have been extensively studied in order to determine their structure and thermal stability, as well as the motion and disorder of the molecules. We have prepared stable van der Waals-bonded C60/benzene solvates using a novel crystallization technique.[7] NMR shows that there is very little interaction between benzene and the C60 molecules and that the rotation of C60 at room temperature is not hindered. Three benzene species have been found in the solvate: two, nearly freely mobile, in wide and narrow porous structures, and the third constrained by C60 molecules in lattice defects. The C60.2CHBr3 solvate is hexagonal with an alternating layer arrangement (Figure
4). Bromoform resides in the interstices formed by an AAA packing of C60
layers.[8,9] Its presence does not hinder the motion of the fullerene cages, as
13C NMR linewidths are comparable with those for pure oxygen-free C60. In the 193–295K temperature range the C60 molecules undergo fast isotropic reorientation.
Figure 4
|
![]()
|
Krätschmer, W., Lamb, L. D., Fostiropoulos, K. & Huffman, D. R. Solid C60 - a new form of carbon. Nature 347, 354-358 (1990). |
|
| [2] | Kroto, H. W., Allaf, A. W. & Balm, S. P. C60: buckminsterfullerene. Chem. Rev. 91, 1213-1235 (1991). |
| [3] | Kolodziejski, W., Corma, A., Barras, J. & Klinowski, J. Detection of fulleroid sites in fullerene-60 by high-resolution solid-state 1H NMR. J. Phys. Chem. 99, 3365-3370 (1995). |
| [4] | Kolodziejski, W. & Klinowski, J. 13C->1H and 1H->13C cross-polarization NMR in toluene-solvated fullerene-70. Chem. Phys. Lett. 247, 507-509 (1995). |
| [5] | Collins, C., Kolodziejski, W., Foulkes, J. & Klinowski, J. High-resolution 2H solid-state NMR study of water intercalated in fullerene C60. Chem. Phys. Lett. 289, 338-340 (1998). |
| [6] | He, H. Y., Teixeira-Dias, J., Foulkes, J. & Klinowski, J. 13C solid-state MAS NMR studies of the low temperature phase transition in fullerene C60. Phys. Chem. Chem. Phys. 2, 2651-2654 (2000). |
| [7] | He, H. Y., Barras, J., Foulkes, J. & Klinowski, J. Solid-state NMR studies of fullerene C60/benzene solvates. J. Phys. Chem. B101, 117-122 (1997). |
| [8] | Collins, C., Foulkes, J., Bond, A. & Klinowski, J. Crystalline C60 . CHBr3 solvate: a solid-state study. Phys. Chem. Chem. Phys. 1, 5323–5326 (1999). |
| [9] | Collins, C., Foulkes, J., Bond, A. D. & Klinowski, J. Crystalline C60 . 2CHBr3 solvate: A solid-state study. Phys. Chem. Chem. Phys. 1, 5323-5326 (1999). |
| [10] | Korobov, M. V., Mirakian, A. L., Avramenko, N. V., Valeev, E. F., Neretin, I. S., Slovokhotov, Y. L., Smith, A. L., Olofsson, G. & Ruoff, R. S. C-60 . bromobenzene solvate: crystallographic and thermochemical studies and their relationship to C60 solubility in bromobenzene. J. Phys. Chem. B102, 3712-3717 (1998). |
| [11] | Meidine, M. F., Hitchcock, P. B., Kroto, H. W., Taylor, R. & Walton, D. R. M. Single crystal X-ray structure of benzene solvated C60. J. Chem. Soc., Chem. Commun., 1534-1537 (1992). |
| [12] | Balch, A. L., Lee, J. W., Noll, B. C. & Olmstead, M. M. Disorder in a crystalline form of Buckminsterfullerene - C60.4C6H6. J. Chem. Soc., Chem. Commun., 56-58 (1993). |