|
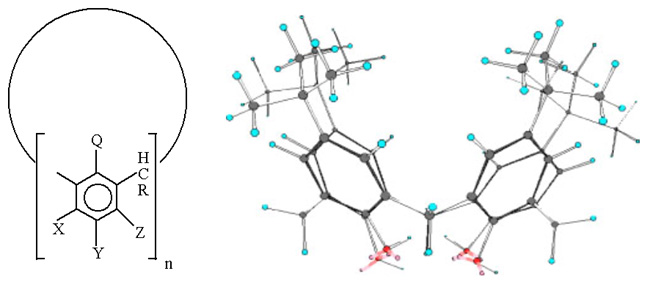
Calixarenes[1,2] are large cyclic molecules made up of phenol units linked via alkylidene groups and containing large cavities of molecular dimensions. Their bowl-like structure (Latin calix = bowl) allows them to sequester a variety of other molecules, which is of great interest to guest-host chemistry, purification, chromatography, storage and slow release of drugs. In the general chemical formula given below typically X = Y = Z = H and Q = OH.
Fine control of the size of the molecule (by changing the value of n) and the introduction of X, Y, Z, Q and R functional groups make it possible to “tailor” calixarenes for a variety of chemical applications: as catalysts, ligands and molecular hosts for neutral and charged inorganic and organic species. Chiral calixarenes can also be prepared. Their sequestering properties are exceptional, while appropriate substitution renders the cavities shape-selective and suitable for molecular recognition. Calixarenes and their complexes are ideal as models in inorganic chemistry (water-soluble salts forming layered clay-like structures) and biochemistry (bioreceptors, ionophores and iron complexes capable of transporting molecular oxygen). Chain-chain interactions and hydrogen bonding involving OH groups play a crucial role in their supramolecular chemistry of such complexes. We have examined a number of simple calixarenes and their inclusion complexes with small organic molecules, such as acetone, benzene and acetonitrile.[3-8] Crystal structures were solved, refined and discussed in terms of molecular interactions. The effect of the cavity size was also taken into account. The change of structure with temperature and the decomposition of the complexes were investigated using thermogravimetric analysis (TGA). Calixarene crystals were examined by a variety of spectroscopic techniques, including infrared (IR) microscopy and high-resolution solid-state nuclear magnetic resonance (NMR). IR microscopy allows one to study small crystals as they are, thus avoiding the KBr pellet or nujol techniques, which interfere in the material structure. The method gives multidimensional IR images, which can be viewed as various cross-sections, for example as two-dimensional plots at particular spectral frequency. This spectroscopic method is very useful in the study of hydrogen bonding.
The objectives of the project are: 1. To characterize the intra- and intermolecular forces in the solid state, using infrared and nuclear magnetic resonance spectroscopies, and to elucidate their role in complex formation. The crystal structures will be determined by single-crystal X-ray diffraction.
2. To characterize molecular dynamics in calixarene complexes.
3. To design new complexes to be applied in chemistry, biochemistry and pharmaceutical industry.
|
![]()
|
Vicens, J. & Böhmer, V. (eds.) Calixarenes: A Versatile Class of Macrocyclic Compounds (Kluwer Academic Publishers, Dordrecht, 1991). |
|
| [2] | Gutsche, C. D. in Monographs in Supramolecular Chemistry (ed. Stoddart, J. F.) (The Royal Society of Chemistry, Cambridge, 1989). |
| [3] | Benevelli, F., Kolodziejski, W., Wozniak, K. & Klinowski, J. Solid-state NMR studies of alkali metal ion complexes of p-tertbutyl-calixarenes. Chem. Phys. Lett. 308, 65-70 (1999). |
| [4] | Benevelli, F., Bond, A., Duer, M. & Klinowski, J. Chloroform encapsulated in p-tert-butylcalix[4]arene: Structure and dynamics. Phys. Chem. Chem. Phys. 2, 3977-3981 (2000). |
| [5] | Pietraszkiewicz, M., Pietraszkiewicz, O., Kolodziejski, W., Wozniak, K., Feeder, N., Benevelli, F. & Klinowski, J. X-ray diffraction and 13C solid-state NMR studies of the dimethylformamide solvate of tetra(C- undecyl)calix[4]resorcinarene. J. Phys. Chem. B 104, 1921-1926 (2000). |
| [6] | Benevelli, F., Kolodziejski, W., Wozniak, K. & Klinowski, J. Complexation behaviour of p-tert-butylcalix[4]arene and p-tert- butylcalix[6]arene towards acetone. Phys. Chem. Chem. Phys. 3, 1762-1768 (2001). |
| [7] | Benevelli, F., Klinowski, J., Bitter, I., Grun, A., Balazs, B. & Toth, G. Stereochemistry of capped calix[4]arenes in liquid and solid phase by NMR spectroscopy. J. Chem. Soc., Perkin Trans. 2, 1187-1192 (2002). |
| [8] | Kuzmicz, R., Dobrzycki, L., Wozniak, K., Benevelli, F., Klinowski, J. & Kolodziejski, W. X-ray diffraction and13C solid-state NMR studies of the solvate of tetra(C-undecyl) calix[4]resorcinarene with dimethylacetamide. Phys. Chem. Chem. Phys. 4, 2387-2391 (2002). |
| [9] | van Wageningen, A. M. A., Verboom, W., Zhu, X., Ripmeester, J. A. & Reinhoudt, D. N. Solid state NMR spectroscopy of calix[4]arene-based carceplexes. Supramol. Chem. 9, 31-36 (1998). |
| [10] | Brouwer, E. B., Enright, G. D. & Ripmeester, J. A. Solid-state NMR and diffraction studies of a tunable p-tert-butylcalix[4] arene guest structures. J. Am. Chem. Soc. 119, 5404-5412 (1997). |